Chemistry, 20.01.2021 17:20, Priskittles
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emissions such as carbon monoxide, CO(g), into carbon dioxide, CO2(g). The uncatalyzed reaction is represented by the balanced equation below.2CO(g) O2(g) 2CO2(g) +heat. determine the mass of O2(g) required to completely react with 784g moles of CO(g) during this reaction.

Answers: 2
Other questions on the subject: Chemistry



Do you know the correct answer?
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emission...
Questions in other subjects:





SAT, 09.02.2022 09:50

English, 09.02.2022 09:50



Mathematics, 09.02.2022 09:50


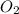 will be required to completely react with 784g moles of CO(g) during this reaction.
will be required to completely react with 784g moles of CO(g) during this reaction.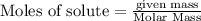
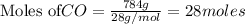
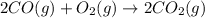
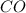 require = 1 mole of
require = 1 mole of 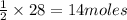 of
of