
100 points if you help quickly
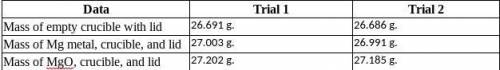
Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
Determine the percent yield of MgO for your experiment for each trial.
Trial 1:
Trial 2:
Determine the average percent yield of MgO for the two trials.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 07:30, Ayyyyeeeeeeewuzgud
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1

Chemistry, 23.06.2019 15:30, payshencec21
Which of the following is an example of a crystalline solid
Answers: 1
Do you know the correct answer?
100 points if you help quickly
Magnesium is the limiting reactant in this experiment. Calculate the...
Questions in other subjects:


Mathematics, 29.08.2019 03:10

Mathematics, 29.08.2019 03:10

Mathematics, 29.08.2019 03:10

History, 29.08.2019 03:10



Mathematics, 29.08.2019 03:10








