Chemistry, 20.01.2021 04:40, alarimer3695
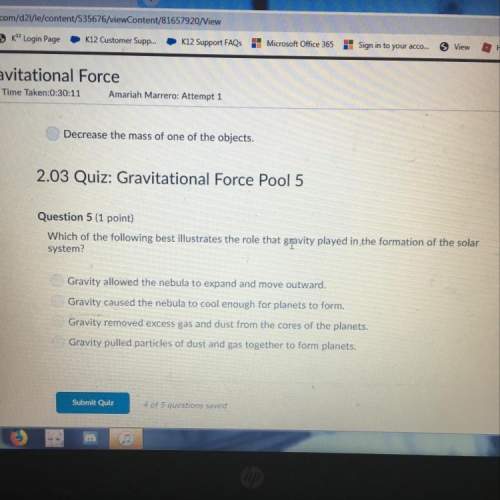
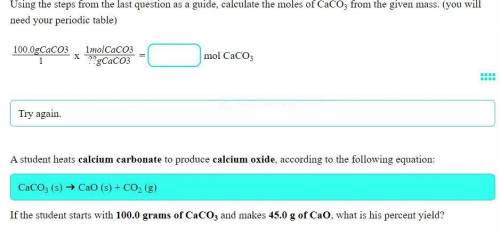
A student heats calcium carbonate to produce calcium oxide, according to the following equation:
CaCO3 (s) ➔ CaO (s) + CO2 (g)
If the student starts with 100.0 grams of CaCO3 and makes 45.0 g of CaO, what is his percent yield?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2



Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
A student heats calcium carbonate to produce calcium oxide, according to the following equation:
Ca...
Questions in other subjects:

Mathematics, 05.05.2020 01:56

Mathematics, 05.05.2020 01:56

History, 05.05.2020 01:56


English, 05.05.2020 01:56



Mathematics, 05.05.2020 01:56


English, 05.05.2020 01:56