
Chemistry, 20.01.2021 04:10, savannahvargas512
Explain the trend you see in your graph and explain why this occurs at a particle level what is happening when the volume or pressure increases what does the particles do within the sample explain this concept in detail
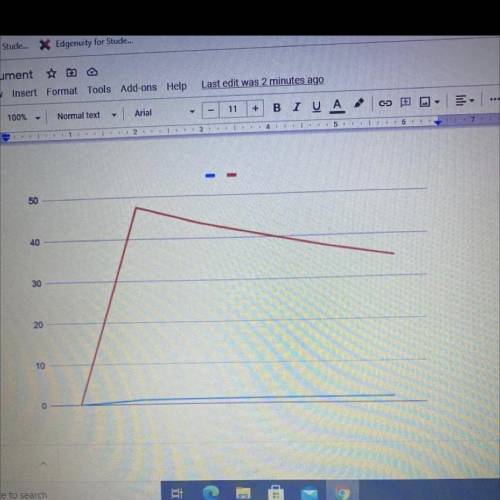

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
Do you know the correct answer?
Explain the trend you see in your graph and explain why this occurs at a particle level what is happ...
Questions in other subjects:

Mathematics, 12.05.2021 20:40



Mathematics, 12.05.2021 20:40

English, 12.05.2021 20:40

Mathematics, 12.05.2021 20:40


Business, 12.05.2021 20:40







