Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
Do you know the correct answer?
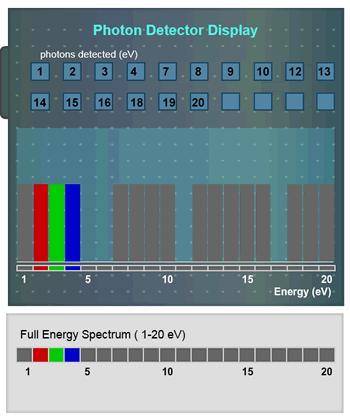
Suppose the innermost orbit an electron can occupy has an energy level of -19 eV. Based on the absor...
Questions in other subjects:

English, 06.09.2019 18:10

Chemistry, 06.09.2019 18:10

English, 06.09.2019 18:10




History, 06.09.2019 18:10


Mathematics, 06.09.2019 18:10