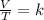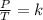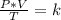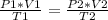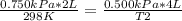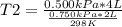Chemistry, 20.01.2021 01:50, KylaChanel4756
A sample of gas has a volume of 2.00 L and a pressure of 0.750 kPa when its
temperature is 25°C. If the volume is expanded to 4.00 L and the pressure reduced to
0.500 kPa, what must the temperature become?
379°C
397°C
379 K
397K

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, hannah2718
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1


Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Do you know the correct answer?
A sample of gas has a volume of 2.00 L and a pressure of 0.750 kPa when its
temperature is 25°C. If...
Questions in other subjects:


Mathematics, 25.01.2022 02:40

English, 25.01.2022 02:40

Mathematics, 25.01.2022 02:40

Mathematics, 25.01.2022 02:40

Mathematics, 25.01.2022 02:40

Mathematics, 25.01.2022 02:40

History, 25.01.2022 02:40


Physics, 25.01.2022 02:40