d. none of the above (all have the same kinetic energy)
Explanation:
The kinetic theory of gases states that the molecules of an ideal gas experience a constant random motion.
At standard temperature and pressure (STP), the kinetic energy of an ideal gas such as hydrogen, argon, neon, sodium, oxygen, helium, magnesium, beryllium, nitrogen, carbon, fluorine, chlorine etc are all the same.
The standard temperature and pressure (STP) of an ideal gas is 273K and 100 kPa.
Hence, all of the gases have the same kinetic energy at standard temperature and pressure (STP).
Kinetic energy can be defined as an energy possessed by an object or body due to its motion.
Mathematically, kinetic energy is given by the formula;
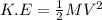
Where, K.E represents kinetic energy measured in Joules.
M represents mass measured in kilograms.
V represents velocity measured in metres per seconds square.














