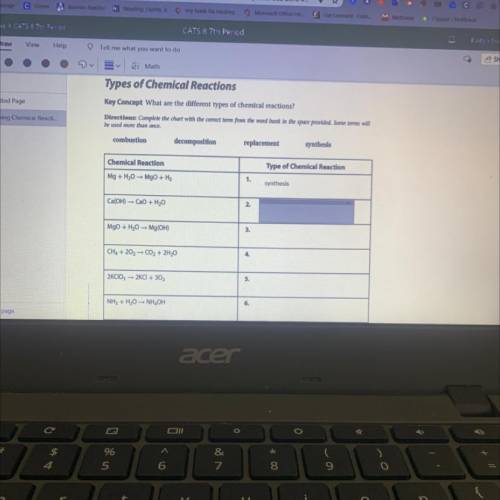
Ca(OH) =CaO + H2O
2.
MgO + H2O Mg(OH)
3.
CH4 + 202 - CO2 + 2H20
4.
2K...

Chemistry, 19.01.2021 21:10, Crtive5515
Ca(OH) =CaO + H2O
2.
MgO + H2O Mg(OH)
3.
CH4 + 202 - CO2 + 2H20
4.
2KCIO3 -- 2KCI+ 302
5.
NH3 + H20 NH OH
6.
Zn + 2HCI ZnCl2 + H2
7.
Add page
acer

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


Do you know the correct answer?
Questions in other subjects:

Chemistry, 30.09.2019 00:10




Mathematics, 30.09.2019 00:10

History, 30.09.2019 00:10

Social Studies, 30.09.2019 00:10

Mathematics, 30.09.2019 00:10

Mathematics, 30.09.2019 00:10






