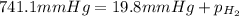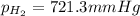Chemistry, 19.01.2021 21:10, alivas6618
(d) In the laboratory, the temperature is 295 K and the total pressure in the gas-collecting tube is 741.2 mmHg . If the vapor pressure of water at 295 K is 19.8 mmHg , determine the pressure of the H2(g) in the gas-collecting tube.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, martinez6221
Forests and meadows are often cut down to make way for farms or large number of new homes. what are some of the elements of ecosystems that are lost when plants in these areas are removed?
Answers: 2

Do you know the correct answer?
(d) In the laboratory, the temperature is 295 K and the total pressure in the gas-collecting tube is...
Questions in other subjects:


History, 05.11.2019 03:31

English, 05.11.2019 03:31

Health, 05.11.2019 03:31



Mathematics, 05.11.2019 03:31


History, 05.11.2019 03:31


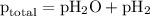
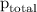 = total pressure of gases = 741.1 mm Hg
= total pressure of gases = 741.1 mm Hg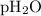 = partial pressure of water = 19.8 mm Hg
= partial pressure of water = 19.8 mm Hg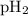 = partial pressure of hydrogen = ?
= partial pressure of hydrogen = ?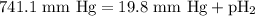
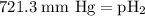
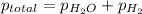
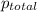 =total pressure of gases = 741.1 mm Hg
=total pressure of gases = 741.1 mm Hg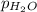 = partial pressure of water = 19.8 mm Hg
= partial pressure of water = 19.8 mm Hg 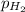 = partial pressure of hydrogen = ?
= partial pressure of hydrogen = ?