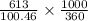Chemistry, 19.01.2021 19:30, holycow4916
A chemist dissolves 613 mg of pure perchloric acid in enough water to make up 360 mL of solution. Calculate the pH of the solution. Round your answer to 3 significant decimal places.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
Do you know the correct answer?
A chemist dissolves 613 mg of pure perchloric acid in enough water to make up 360 mL of solution. Ca...
Questions in other subjects:

World Languages, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50

History, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50

English, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50



Mathematics, 26.02.2021 22:50


 .................1
.................1