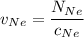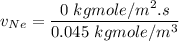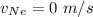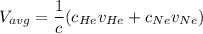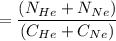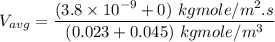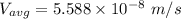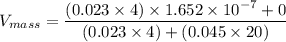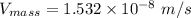Chemistry, 19.01.2021 05:30, Jadamachado45
In a gas-phase diffusion mass-transfer process, the steady-state flux of helium in a binary mixture of helium and neon is 3.8 x 10-9 kgmole/cm2 s, and the flux of neon is 0. At a particular point in the diffusion space, the concentration of helium is 0.023 kgmole/m3 and the concentration of neon is 0.045 kgmole/m3 . Estimate the individual net velocities of helium and neon along the direction of mass transfer, the average molar velocity, and the average mass velocity.''

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
Do you know the correct answer?
In a gas-phase diffusion mass-transfer process, the steady-state flux of helium in a binary mixture...
Questions in other subjects:

Biology, 16.07.2019 17:30




Health, 16.07.2019 17:30


Biology, 16.07.2019 17:30




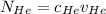
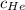 = concentration of Helium
= concentration of Helium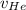 = net velocity of Helium
= net velocity of Helium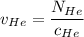
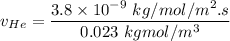
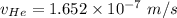
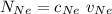
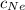 = Concentration of neon
= Concentration of neon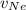 = net velocity of neon species
= net velocity of neon species