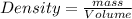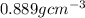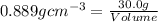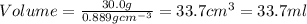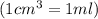A chemistry student needs 30.0 of tetrahydrofuran for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of tetrahydrofuran is 0.889 g x cm^-3 . Calculate the volume of tetrahydrofuran the student should pour out.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Do you know the correct answer?
A chemistry student needs 30.0 of tetrahydrofuran for an experiment. By consulting the CRC Handbook...
Questions in other subjects:


Mathematics, 28.01.2020 09:31


Mathematics, 28.01.2020 09:31


History, 28.01.2020 09:31



Chemistry, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31