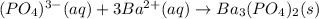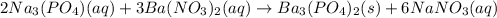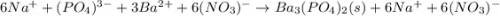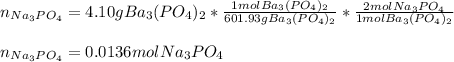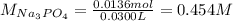Chemistry, 18.01.2021 22:00, cyaransteenberg
30.0 mL of Na3PO4(aq) is reacted with excess 2.00 M Ba(NO3)2(aq). A precipitate of Ba3(PO4)2(s) is formed. The precipitate is filtered and dried to constant mass. The mass of precipitate produced is 4.10 g. (a) Write the balanced net ionic equation of the reaction. (b) How many moles of Na3PO4 reacted

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, alexisdiaz365
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Do you know the correct answer?
30.0 mL of Na3PO4(aq) is reacted with excess 2.00 M Ba(NO3)2(aq). A precipitate of Ba3(PO4)2(s) is f...
Questions in other subjects:


English, 05.07.2019 20:20

Physics, 05.07.2019 20:20


Law, 05.07.2019 20:20


History, 05.07.2019 20:20