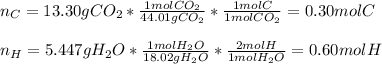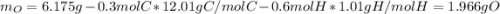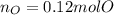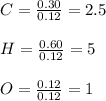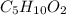Chemistry, 18.01.2021 21:40, 5924000264
A 6.175 gram sample of an organic compound containing only C, H, and O is analyzed by combustion analysis and 13.30 g CO2 and 5.447 g H2O are produced. In a separate experiment, the molar mass is found to be 102.1 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
Do you know the correct answer?
A 6.175 gram sample of an organic compound containing only C, H, and O is analyzed by combustion ana...
Questions in other subjects:



Mathematics, 16.10.2020 07:01


History, 16.10.2020 07:01




Social Studies, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01