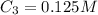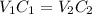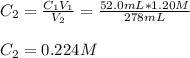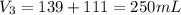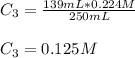Chemistry, 18.01.2021 21:10, tylermorehead1
A 52.0 mL portion of a 1.20 M solution is diluted to a total volume of 278 mL. A 139 mL portion of that solution is diluted by adding 111 mL of water. What is the final concentration

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2

Chemistry, 23.06.2019 02:00, Turtlelover05
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 05:30, brianrodriguez2005
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b. cns c. ans d. pns
Answers: 1
Do you know the correct answer?
A 52.0 mL portion of a 1.20 M solution is diluted to a total volume of 278 mL. A 139 mL portion of t...
Questions in other subjects:

Mathematics, 06.11.2020 01:00


History, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Social Studies, 06.11.2020 01:00



Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00