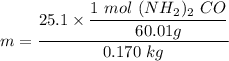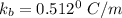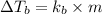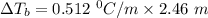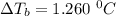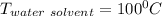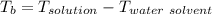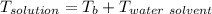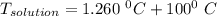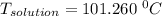Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 23.06.2019 06:00, kristine2424
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
Do you know the correct answer?
A solution is prepared by dissolving 25.1 g urea, (NH2)2CO, in 170.9 g water. Calculate the boiling...
Questions in other subjects:

Chemistry, 07.07.2021 03:50



Mathematics, 07.07.2021 03:50


Mathematics, 07.07.2021 04:00


Mathematics, 07.07.2021 04:00


Mathematics, 07.07.2021 04:00