
Chemistry, 18.01.2021 18:10, mallorytaylor279
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydroxide solution of concentration 0.15 mol/dm3.
The equation which represents the reaction is:
HCl + NaOH > NaCl + H2O
Calculate the concentration of hydrochloric acid in mol/dm3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
2.In a titration, 25.00 cm3 of a solution of hydrochloric acid reacted with 18.40 cm3 of sodium hydr...
Questions in other subjects:

Geography, 30.06.2019 14:20

Mathematics, 30.06.2019 14:20

History, 30.06.2019 14:20

Mathematics, 30.06.2019 14:20

Mathematics, 30.06.2019 14:20


Physics, 30.06.2019 14:20


Biology, 30.06.2019 14:20

Mathematics, 30.06.2019 14:20


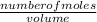 =
= 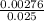 = 0.11mol/dm³
= 0.11mol/dm³



