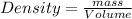Chemistry, 18.01.2021 14:00, melanie687
A student determines the density of an unknown liquid by weighing a sample of known volume. The reported density is found by the instructor to be significantly higher than it should be. Which of the following experimental errors could account for this result?
A. The pipet used was filled to a level above the calibration mark.
B. The balance was read incorrectly, and the recorded sample mass was lower than the true sample mass.
or
C. A part of the sample was spilled from the beaker before it was weighed.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
Do you know the correct answer?
A student determines the density of an unknown liquid by weighing a sample of known volume. The repo...
Questions in other subjects:






History, 12.03.2020 20:24



Mathematics, 12.03.2020 20:24