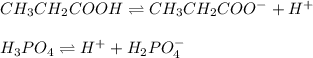Chemistry, 18.01.2021 14:00, zalyndevola
Propionic acid (CH3CH-COOH) has a K, 1.3 x 10^-5 and phosphoric acid (H3PO2) has a Ka = 7.5 x 10^-3
Choose the conjugate base for each.
A. CH3CH2COO2- for CH3CH2COOH; HPO4 2- for H3PO4
B. CH3CH2CO- for CH3CH2COOH; H2PO3 - for H3PO4
C. CH3CH2COOH2 for CH3CH2COOH; H4PO4 for H3PO4
D. CH3CH2COO- for CH3CH2COOH; H2PO4 - for H3PO4

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
Do you know the correct answer?
Propionic acid (CH3CH-COOH) has a K, 1.3 x 10^-5 and phosphoric acid (H3PO2) has a Ka = 7.5 x 10^-3...
Questions in other subjects:

History, 10.01.2020 05:31




History, 10.01.2020 05:31

Biology, 10.01.2020 05:31


Social Studies, 10.01.2020 05:31


Mathematics, 10.01.2020 05:31