
Chemistry, 18.01.2021 14:00, superbatman9193
(3)
Calculate the entropy change when a sample of argon at 25 °C and 1.00 atm in a container
of volume 500 cm3 is compressed to 50 cm3 and cooled to -25 °C.
For argon, Cpm = 20.786) K-Imol-1. Assume that argon behaves perfectly.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1



Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
Do you know the correct answer?
(3)
Calculate the entropy change when a sample of argon at 25 °C and 1.00 atm in a container
o...
o...
Questions in other subjects:

Mathematics, 12.12.2020 20:00


Mathematics, 12.12.2020 20:00



Mathematics, 12.12.2020 20:00

English, 12.12.2020 20:00



Mathematics, 12.12.2020 20:10



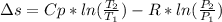
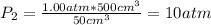
![\Delta s =20.786\frac{J}{mol*K} *ln[\frac{(-25+273)K}{(25+273)K} ]-8.3145\frac{J}{mol*K}*ln(\frac{10atm}{1atm} )\\\\\Delta s=-23.0\frac{J}{mol*K}](/tpl/images/1043/0995/45d4a.png)




