
Hydrobromic acid dissolves solid iron according to the following reaction:
Fe(s)+2HBr(aq)→FeBr2(aq)+H2(g)
What mass of HBr (in g) would you need to dissolve a 2.8-g pure iron bar on a padlock?
What mass of H2 would be produced by the complete reaction of the iron bar?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 23.06.2019 14:00, jadkins842
Ahas distinct properties and composition that never vary.
Answers: 1

Chemistry, 23.06.2019 16:00, jakebuttone
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a. no b. n2o4 c. no2 d. n4o8
Answers: 1

Chemistry, 23.06.2019 19:30, kortetsosie8813
⁉️how many kj of energy would be needed to convert 150. g of ammonia to vapor at its boiling point? ⁉️(ammonia’s heat of vaporization is 1.38 kj/g
Answers: 1
Do you know the correct answer?
Hydrobromic acid dissolves solid iron according to the following reaction:
Fe(s)+2HBr(aq)→FeBr2(aq...
Questions in other subjects:



Mathematics, 15.01.2020 22:31

Mathematics, 15.01.2020 22:31

Mathematics, 15.01.2020 22:31



Mathematics, 15.01.2020 22:31



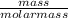
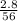 = 0.05mole
= 0.05mole 




