
Chemistry, 18.01.2021 02:50, zriggi2528
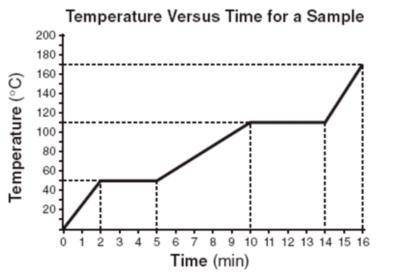
Given the heating curve of a solid being heated at a constant rate, how much total time was required to boil this substance after it reached its melting point?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
Do you know the correct answer?
Given the heating curve of a solid being heated at a constant rate, how much total time was required...
Questions in other subjects:


Physics, 07.03.2021 14:50


Social Studies, 07.03.2021 15:00

Mathematics, 07.03.2021 15:00

Chemistry, 07.03.2021 15:00



Mathematics, 07.03.2021 15:00

Physics, 07.03.2021 15:00






