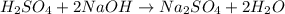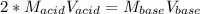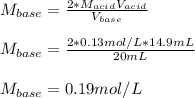Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
Do you know the correct answer?
PLEASE HELP WHICH ONE IS IT AND CAN YOU EXPLAN HOW YOU GOT THE ANSWERS TY!!!
In a titration, a 20 m...
Questions in other subjects:


History, 20.05.2020 03:00

Mathematics, 20.05.2020 03:00


Mathematics, 20.05.2020 03:00

Geography, 20.05.2020 03:00

Mathematics, 20.05.2020 03:00

English, 20.05.2020 03:00

Mathematics, 20.05.2020 03:00