
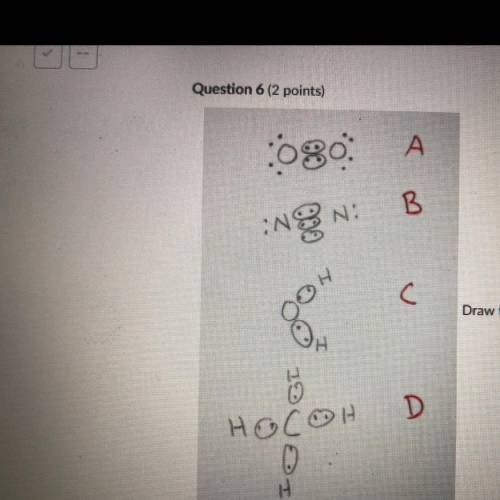
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select
the answer that best describes which drawing is wrong and why. Note I drew circles
around electrons that are participating in covalent bonding. This is normally not done
but for the purpose of this test the circled electrons are fine.
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the
Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 02:30, ineedhelp2285
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
Do you know the correct answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions in other subjects:

History, 19.11.2020 01:10


Chemistry, 19.11.2020 01:10

Mathematics, 19.11.2020 01:10


Mathematics, 19.11.2020 01:10

Mathematics, 19.11.2020 01:10

Chemistry, 19.11.2020 01:10


Mathematics, 19.11.2020 01:10






