
Chemistry, 16.01.2021 17:10, justapointie
Sodium bicarbonate (NaHCO3) is commercially known as baking soda. How many grams and atoms of oxygen are there in 15 grams of sodium bicarbonate? The molar mass of NaHCO3 is 84.01 g/mol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
Do you know the correct answer?
Sodium bicarbonate (NaHCO3) is commercially known as baking soda. How many grams and atoms of oxygen...
Questions in other subjects:

Chemistry, 27.01.2021 22:30


Mathematics, 27.01.2021 22:30



Spanish, 27.01.2021 22:30

Mathematics, 27.01.2021 22:30


Physics, 27.01.2021 22:30

History, 27.01.2021 22:30


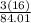 x 15 = 8.57g
x 15 = 8.57g



