
Chemistry, 16.01.2021 14:40, jakeyywashere
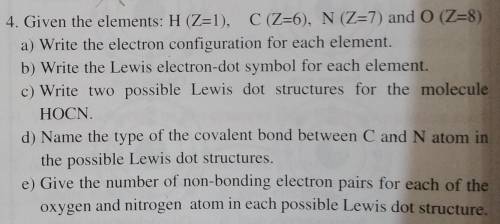
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration for each element.
b) Write the Lewis electron-dot symbol for each element.
c) Write two possible Lewis dot structures for the molecule
HOCN.
d) Name the type of the covalent bond between C and N atom in
the possible Lewis dot structures.
e) Give the number of non-bonding electron pairs for each of the
oxygen and nitrogen atom in each possible Lewis dot structure

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 08:30, vanessadaniellet21
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
Do you know the correct answer?
4. Given the elements: H (Z=1), C (Z=6), N (Z=7) and 0 (Z=8)
a) Write the electron configuration fo...
Questions in other subjects:

Mathematics, 25.11.2020 19:00


Mathematics, 25.11.2020 19:00


Mathematics, 25.11.2020 19:00




Arts, 25.11.2020 19:00

Biology, 25.11.2020 19:00






