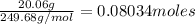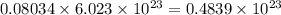Will mark the brainiest later for correct answers! Please show work.
Mass C12H22O11 = 14.08g
...

Will mark the brainiest later for correct answers! Please show work.
Mass C12H22O11 = 14.08g
Convert grams to moles:
Convert grams to molecules:
Mass NaCI: 17.75g
Convert grams to molecules:
Convert grams to formula units:
Copper (II) Sulfate Pentahydrate
Chemical Formula: CuSO4.5H2O
Mass: 20.06g
Convert grams to moles:
Formula Units:

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, mahoganyking16
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 21.06.2019 16:00, lizzyhearts
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2
Do you know the correct answer?
Questions in other subjects:




Chemistry, 02.09.2019 10:30


Mathematics, 02.09.2019 10:30

Mathematics, 02.09.2019 10:30

Mathematics, 02.09.2019 10:30

Mathematics, 02.09.2019 10:30


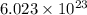 of particles and weighs equal to its molecular mass.
of particles and weighs equal to its molecular mass.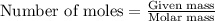
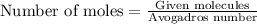
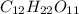 =
= 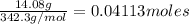
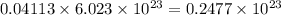
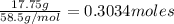
 =
= 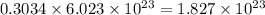
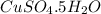 =
=