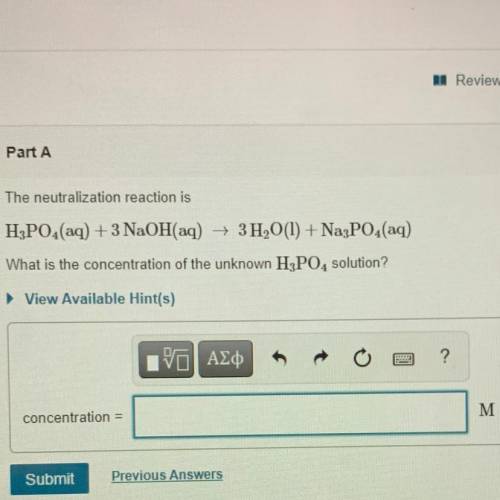
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the conc...

Chemistry, 15.01.2021 07:00, SauceyNaee
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the concentration of the unknown H3PO4 solution?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 02.02.2021 03:00



History, 02.02.2021 03:00


Mathematics, 02.02.2021 03:00

Advanced Placement (AP), 02.02.2021 03:00

Mathematics, 02.02.2021 03:00


Mathematics, 02.02.2021 03:00






