

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2
Do you know the correct answer?
4. Calculate the molar mass of Fe2(SO4)3. Then calculate the mass of iron(III) sulfate of 4.05X1023...
Questions in other subjects:

Mathematics, 23.02.2021 04:20

History, 23.02.2021 04:20



Mathematics, 23.02.2021 04:20

Social Studies, 23.02.2021 04:20



Chemistry, 23.02.2021 04:20

English, 23.02.2021 04:20


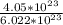 =269
=269



