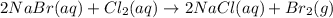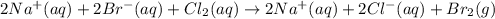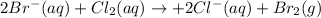Chemistry, 14.01.2021 23:40, nickname0097
A sodium bromide solution is added to a beaker containing aqueous chlorine. What would happen?
A) Write the complete chemical equation
B) Write the dissociated ionic equation
C) Write the net ionic equation
D) List any spectator ions

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
Do you know the correct answer?
A sodium bromide solution is added to a beaker containing aqueous chlorine. What would happen?
A) W...
Questions in other subjects:


Mathematics, 12.03.2021 05:10

Social Studies, 12.03.2021 05:10

Mathematics, 12.03.2021 05:10

Mathematics, 12.03.2021 05:10

English, 12.03.2021 05:10




Mathematics, 12.03.2021 05:10