
Chemistry, 14.01.2021 23:00, clashofclans17
50 POINTS PLEASE NO FAKE ANSWERS I REALLY NEED THESE ANSWERED
1. The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl + Ca(NO3)2
How many grams of AgCl are produced from 30.0 grams of CaCl2?
(Molar mass of Ca = 40.078 g/mol, Cl = 35.453 g/mol, O = 15.999 g/mol, Ag = 107.868 g/mol, N = 14.007 g/mol)
19.4 grams
38.8 grams
58.2 grams
77.5 grams
2. The table shows the recipe and the available ingredients for making the maximum possible number of sandwiches.
Making Sandwiches
Recipe for One Sandwich Ingredients Available
2 cheese slices, 1 ham slice, 2 bread slices 12 cheese slices, 10 ham slices, 12 bread slices
If the ingredients represent reactants of a chemical reaction, which of the following represents the leftover reactant?
Two ham slices
Four ham slices
Two cheese slices
Four cheese slices
3. Read the given chemical reaction.
C2H6 + O2 → CO2 + H2O
How many moles of H2O are produced during the complete combustion of 1.4 moles of C2H6?
2.8 moles
4.2 moles
5.6 moles
7.0 moles
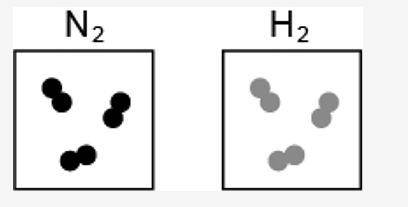
4. The image represents the reaction between a certain number of molecules of N2 and H2.
[IMAGE INCLUDED]
If the maximum possible amount of NH3 is formed during the reaction, what is the leftover reactant?
One molecule of N2
One molecule of H2
Two molecules of N2
Two molecules of H2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
Do you know the correct answer?
50 POINTS PLEASE NO FAKE ANSWERS I REALLY NEED THESE ANSWERED
1. The following reaction shows calci...
Questions in other subjects:

Mathematics, 04.11.2019 15:31

Mathematics, 04.11.2019 15:31

English, 04.11.2019 15:31







Biology, 04.11.2019 15:31






