
Look at the following enthalpy diagram. Select all that apply.
A.)The products have more energy than the reactants.
B.)This is an addition reaction.
C.)A large activation energy is required for this reaction to take place.
D.)The products are more stable than the reactants.
E.)This is a substitution reaction.
You may have more than one answer.
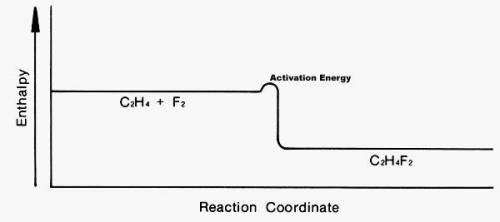

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3


Chemistry, 23.06.2019 01:00, Alysssssssssssa
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
Do you know the correct answer?
Look at the following enthalpy diagram. Select all that apply.
A.)The products have more energy tha...
Questions in other subjects:

Mathematics, 27.11.2019 13:31

History, 27.11.2019 13:31


Mathematics, 27.11.2019 13:31



Biology, 27.11.2019 13:31

Biology, 27.11.2019 13:31


English, 27.11.2019 13:31






