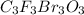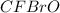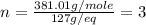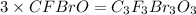Chemistry, 09.12.2019 03:31, krissymonae
What is the molecular formula of a compound if its empirical formula is cfbro and its molar mass is 381.01 g/mol?
a. cfbro
b. c2f2br2o2
c. c3f3br3o3
d. c2f3br2o3
e. c4f4br4o me

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
Do you know the correct answer?
What is the molecular formula of a compound if its empirical formula is cfbro and its molar mass is...
Questions in other subjects:

Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50





Mathematics, 12.12.2020 16:50