
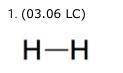
H-H Two side-by-side letter H's connected by a short, horizontal line segment. The Lewis structure for a hydrogen molecule, H2, is shown. The line segment in the structure shows that the two atoms (2 points) share one valence electron. share two valence electrons. have one unshared electron. have two unshared electrons.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Do you know the correct answer?
H-H Two side-by-side letter H's connected by a short, horizontal line segment. The Lewis structure f...
Questions in other subjects:

Mathematics, 20.07.2019 14:30







History, 20.07.2019 14:30


Mathematics, 20.07.2019 14:30






