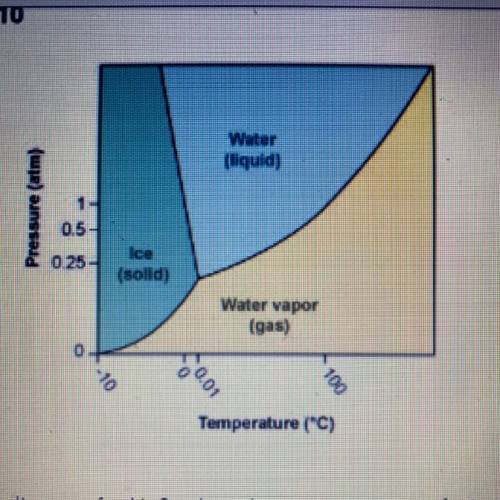
Using the phase diagram for H20, what phase is water in at 1 atm pressure
and 150°C?
A....


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
Do you know the correct answer?
Questions in other subjects:




Mathematics, 30.11.2020 17:30



History, 30.11.2020 17:30

History, 30.11.2020 17:30

Mathematics, 30.11.2020 17:30