Task 4
3.00 moles of lead nitrate and excess potassium iodidq are combined in water to
form l...

Task 4
3.00 moles of lead nitrate and excess potassium iodidq are combined in water to
form lead iodide and potassium nitrate. The reaction is described by the following
chemical equation:
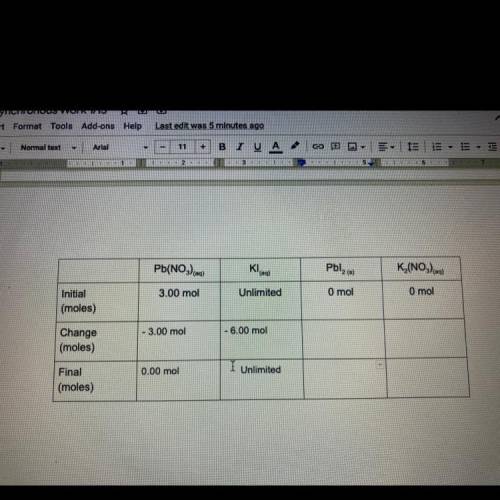
Pb(NO3) eq) + 2Kleg) Pblzco + K (NO)
Fill out the following table to find out how many moles of products are formed,
and how many of the reactants remain after the reaction has taken place. The boxes in
the final row are all mole to mole conversions, and can be found by using your molar
ratios and the moles of lead nitrate at the beginning of the reaction. It is your choice
how you will arrange these two numbers. If this problem becomes confusing, look back
to task 3, It's the same problem!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
Do you know the correct answer?
Questions in other subjects:




English, 11.02.2021 21:50

Mathematics, 11.02.2021 21:50










