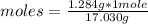Chemistry, 13.01.2021 15:40, anavallesdemiguel2
Calculate the moles of ammonia present in a 1.284 g sample if the molar mass of ammonia is 17.030 g/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
Do you know the correct answer?
Calculate the moles of ammonia present in a 1.284 g sample if the molar mass of ammonia is 17.030 g/...
Questions in other subjects:

Physics, 05.05.2020 05:23

Physics, 05.05.2020 05:23


English, 05.05.2020 05:23

Mathematics, 05.05.2020 05:23


Mathematics, 05.05.2020 05:23

Mathematics, 05.05.2020 05:23