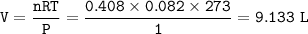Chemistry, 13.01.2021 07:10, tawna6988owtjg6
Trimethylamine, (CH3)2N is a weak base (K6 = 6.3 x 10-5). What volume of this gas, measured at STP, must be dissolved in 2.5 L of solution to give that solution a pOH of 2.50? PLEASE HELP ITS DUE IN 20 mins :

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2


Chemistry, 23.06.2019 10:30, villarrealc1987
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
Do you know the correct answer?
Trimethylamine, (CH3)2N is a weak base (K6 = 6.3 x 10-5). What volume of this gas, measured at STP,...
Questions in other subjects:

Mathematics, 14.12.2021 18:30



Geography, 14.12.2021 18:30

Medicine, 14.12.2021 18:30






![\tt [OH^-]=10^{-2.5}=0.0032=3.2\times 10^{-3}](/tpl/images/1031/5783/b6571.png)
![\tt [OH^-]=\sqrt{Kb.M}\\\\(3.2\times 10^{-3})^2=6.3\times 10^{-5}\times M\\\\M=0.163](/tpl/images/1031/5783/8f3e1.png)