
Chemistry, 13.01.2021 01:50, jokerr5584
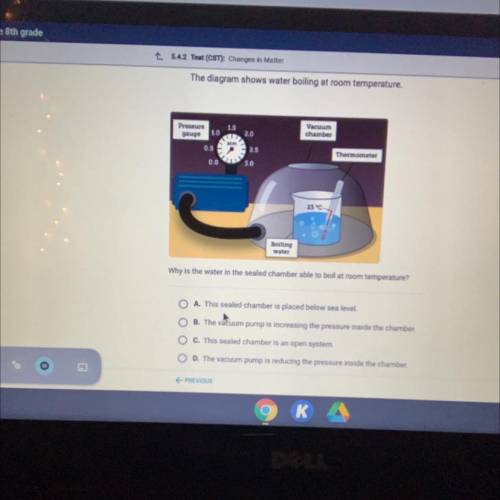
Why is the water in the sealed chamber able to boil at room temperature?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1


Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 07:00, MathChic68
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
Do you know the correct answer?
Why is the water in the sealed chamber able to boil at room temperature?
...
...
Questions in other subjects:



Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01




Mathematics, 23.10.2020 01:01






