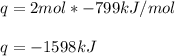Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
Do you know the correct answer?
When 2 moles of Fe(s) react with Cl2(g) to form FeCl3(s) according to the following equation, 799 kJ...
Questions in other subjects:

Mathematics, 05.11.2021 22:30


Mathematics, 05.11.2021 22:30

Mathematics, 05.11.2021 22:30



Arts, 05.11.2021 22:30