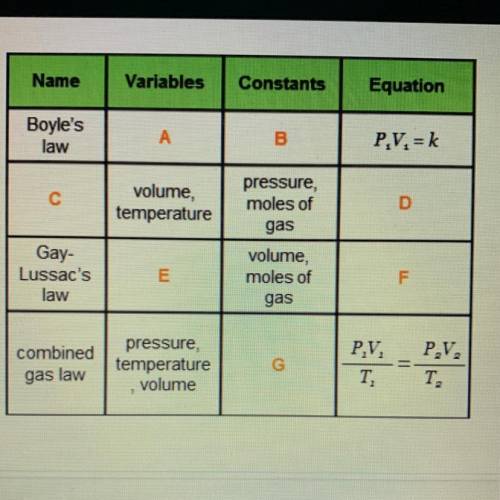
- pressure, volume

Complete the table by filling in the missing information.
Options for A
- pressure, volume
- temperature, pressure
- volume, temperature
Options for B
- pressure, moles of gas
- temperature, moles of gas
- volume, moles of gas
Options for C
- Gay-Lussac’s law
- Charles’s law
- Dalton’s law
Options for D
- V = kT
- V = kP
- V = KP
Options for E
- pressure, volume
- temperature, pressure
- volume, temperature
Options for F
- PT = k
- P = kT
- T = kV
Options for G
- pressure only
- temperature, volume
- number of moles only
- pressure, temperature

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3


Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
Complete the table by filling in the missing information.
Options for A
- pressure, volume
- pressure, volume
Questions in other subjects:

Mathematics, 26.09.2019 01:30

Chemistry, 26.09.2019 01:30

Mathematics, 26.09.2019 01:30

Arts, 26.09.2019 01:30


Mathematics, 26.09.2019 01:30


Social Studies, 26.09.2019 01:30

History, 26.09.2019 01:30






