Chemistry, 11.01.2021 16:10, tawna6988owtjg6
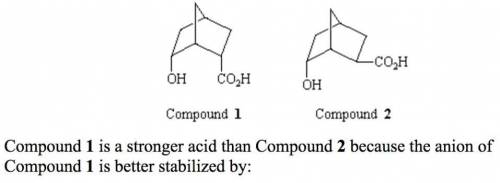
Compound 1 is a stronger acid than Compound 2 because the anion of Compound 1 is better stabilized by:'

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2


Do you know the correct answer?
Compound 1 is a stronger acid than Compound 2 because the anion of Compound 1 is better stabilized b...
Questions in other subjects:




Mathematics, 12.09.2021 22:40

Mathematics, 12.09.2021 22:40


Mathematics, 12.09.2021 22:40

Mathematics, 12.09.2021 22:40