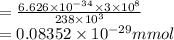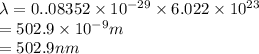Chemistry, 11.01.2021 15:40, vanessacox45
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a carbon-iodine single bond could be broken by absorbing a single photon.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2


Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
Do you know the correct answer?
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a...
Questions in other subjects:


Mathematics, 01.09.2020 03:01

English, 01.09.2020 03:01


Mathematics, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01


Arts, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01