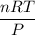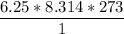Chemistry, 10.01.2021 18:30, kaylaamberd
En un matraz, disponemos de 100 g de gas oxígeno que se encuentran a 1 at de presión y 273 K de temperatura. Calcular : a) el número de moles de gas oxígeno contenidos en el matraz ; b) el número de moléculas de oxígeno ; c) el número de átomos de oxígeno ; d) el volumen ocupado por el oxígeno. Masa atómica del oxígeno = 16.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, chinadoll24
Which of the following is an example of a parasite?
Answers: 3

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
Do you know the correct answer?
En un matraz, disponemos de 100 g de gas oxígeno que se encuentran a 1 at de presión y 273 K de temp...
Questions in other subjects:




Mathematics, 07.04.2020 19:14






Physics, 07.04.2020 19:14