
Chemistry, 09.01.2021 17:00, estefanlionel8678
A group of Chemistry students have 1.0 mole each of two mystery substances, Substance X and Substance Y, at 50° C. Explain why a conclusion about the kinetic energy of each substance is not possible. (Write in short answer form--- 3-5 sentences)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
Do you know the correct answer?
A group of Chemistry students have 1.0 mole each of two mystery substances, Substance X and Substanc...
Questions in other subjects:


Mathematics, 05.01.2021 01:00

Health, 05.01.2021 01:00


Mathematics, 05.01.2021 01:00

Mathematics, 05.01.2021 01:00




History, 05.01.2021 01:00


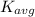 ) of the atoms or molecules contained in the substance. The 6.02 × 10²³ particles (atoms or molecules) within 1.0 mole of each substance can each posses different amount of kinetic energy at any given time. Some particles move very fast while others move very slow such that the kinetic energy of all the particles are only representable by a distribution and a conclusion regarding the different aspects of the kinetic energy distribution of the particles of the substances is not easily possible
) of the atoms or molecules contained in the substance. The 6.02 × 10²³ particles (atoms or molecules) within 1.0 mole of each substance can each posses different amount of kinetic energy at any given time. Some particles move very fast while others move very slow such that the kinetic energy of all the particles are only representable by a distribution and a conclusion regarding the different aspects of the kinetic energy distribution of the particles of the substances is not easily possible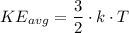
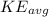 = The average kinetic energy
= The average kinetic energy



