
Chemistry, 08.01.2021 20:10, kinderc9330
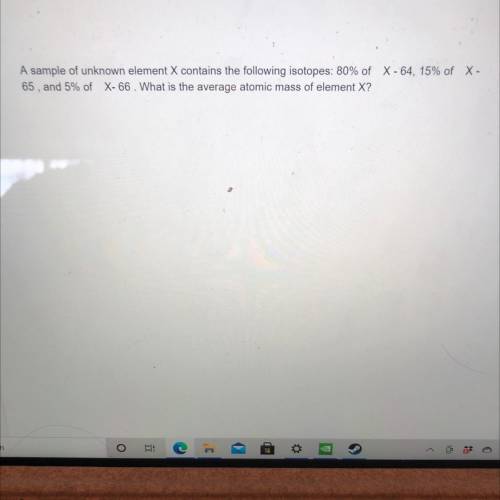
A sample of unknown element X contains the following isotopes: 80% of C -64, 15% of X - 65, and 5% of X-66. What is the average atomic mass of element X? Can somebody help me with this question. Will mark brainliest.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
Do you know the correct answer?
A sample of unknown element X contains the following isotopes: 80% of C -64, 15% of X - 65, and 5% o...
Questions in other subjects:

Mathematics, 24.09.2020 08:01






Geography, 24.09.2020 08:01









