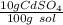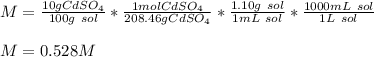Chemistry, 08.01.2021 17:10, danteyoungblood7
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol) by mass? The density of the solution is 1.10 g/mL.
a) 0.528 M
b) 0.436 M
c) 0.479 M
d) 0.048 M
e) 22.9 M

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Do you know the correct answer?
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)...
Questions in other subjects:

History, 21.08.2019 12:30




Social Studies, 21.08.2019 12:30

Mathematics, 21.08.2019 12:30


Health, 21.08.2019 12:30


Geography, 21.08.2019 12:30