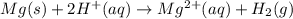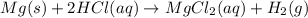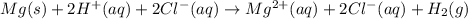Mg(s)+2HCl(aq) → MgCl2(aq)+H2(g)
In an experiment, a student places a small piece of pure Mg(s) into a beaker containing 250.mL of 6.44MHCl(aq) . A reaction occurs, as represented by the equation above.
(a) Write the balanced net ionic equation for the reaction between Mg(s) and HCl(aq) .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2


Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2
Do you know the correct answer?
Mg(s)+2HCl(aq) → MgCl2(aq)+H2(g)
In an experiment, a student places a small piece of pure Mg(s)...
Questions in other subjects:


Mathematics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50

History, 24.02.2021 19:50




History, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50