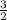Anyone know how to do this?
...

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 11:30, baptistatm51976
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1

Chemistry, 23.06.2019 13:00, 4804344130
Aecosystem is if it can continue to function over long periods of time
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 12.09.2021 23:50


Mathematics, 12.09.2021 23:50


Mathematics, 12.09.2021 23:50


Mathematics, 12.09.2021 23:50

English, 12.09.2021 23:50

Physics, 12.09.2021 23:50

Social Studies, 12.09.2021 23:50