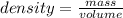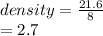Chemistry, 07.01.2021 21:10, hei40563273
a sample of an unknown metal has a mass of 21.6g and volume of an 8.00cm3 calculate the density of the unknown metal

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, lizzyhearts
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 22:00, memeE15
Show transcript transcripts online experiment: atomic structure [the history of atomic theory] this animation will you test rutherford’s hypothesis that it took a great number of shots to actually hit the nucleus because the size of the atom was so much larger than the nucleus.[clipboard and pencil with a diagram of rutherford’s gold foil experiment] we are going to use a cardboard box to represent the atom, a wooden block to represent the nucleus, and marbles to represent the alpha particles. [animated cardboard box, wooden block, and marbles] the box’s measurements are: length = 50 cm, width = 25 cm, and height = 25 cm. the measurements of the block are: length = 10 cm, width = 10 cm, and height is 5 cm. jose and priscilla will conduct the experiment. [animated boy and girl] priscilla put the block in the box on the floor. jose will close his eyes and drop marbles into the box trying to hit the block. priscilla will count the number of times the marbles hit the block and move the block in the box after each hit. [animated block moving inside the box. marble dropping into the box.] here is priscilla’s data of how many times the marble hit the block. out of 100 marble drops or shots, the marble hit the block 32 times. [data table: first column: marble, with rows 1 to 100; column 2: hit, with rows indicating yes or no. last row: total hits] now you can follow the directions in the experiment to present your findings. present your findings when you are finished with the experiment, complete the following data analysis and record your answers in the essay box below. determine the volume of the box and the block. determine the ratio of the block to the box: multiply this number by 100 to turn it into a percent. complete this statement: the volume of the block is percent of the volume of the box. determine the ratio of the number of hits to the number of shots: multiply this number by 100 to turn it into a percent. complete this statement: the block was hit percent of the time. compare the results of step 2 to the results of step 3. are the percentages similar? write a conclusion discussing the following items: based on your findings, do you think rutherford's hypothesis was reasonable? restate rutherford's hypothesis and describe how you tested it. state whether your results support the hypothesis. if they do not, can you suggest some error in experimental procedure (other than general human error) that might explain it? finally, explain how this experiment confirms the nuclear model of the atom and the idea that most of the atom is empty space.
Answers: 1

Chemistry, 23.06.2019 09:00, alyssa0888
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

Chemistry, 23.06.2019 09:00, sammypaige08
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
Do you know the correct answer?
a sample of an unknown metal has a mass of 21.6g and volume of an 8.00cm3 calculate the density of t...
Questions in other subjects:

Chemistry, 04.09.2021 16:50

Law, 04.09.2021 16:50

Mathematics, 04.09.2021 16:50

Mathematics, 04.09.2021 16:50


English, 04.09.2021 16:50




Chemistry, 04.09.2021 16:50